Abstract
Driver mutations of JAK2, CALR and MPL are found in >90% of adults with BCR-ABL1-negative myeloproliferative neoplasms (MPN). In children, the presence of clonal markers ranges between 22 and 40%, and inherited forms of MPD, such as familial erythrocytosis (FE) and hereditary thrombocytosis (HT), are common. Data on the mutational spectrum and biology of childhood MPD are limited. The aims of this study were: a) to evaluate the ability of a next-generation sequencing (NGS)-based 44-gene analysis to better characterize wild type (WT) MPD, and b) to identify non-canonical and/or non-driver mutations in children and adolescents with MPD.
Eighty patients (pts) aged ≤20 years (yrs) at diagnosis of MPD, observed between June 1980 and September 2015, were first investigated with standardized methods for driver mutations of MPN (JAK, MPL, CALR), for genes involved in FE (HRE, EpoR, HIF2α, HIF1α, VHL, PHD1-3, STAT5, LNK, TET2) and HT (THPO, MPL, LNK and TET2). Then, a 44-gene panel providing diagnostic information in myeloid malignancies and in rare inherited erythrocytosis/thrombocytosis (JAK2, CALR, MPL, ASXL1, CBL, C-Kit, CSF3R, CUX1, DNMT3A, ETNK1, EZH2, IDH1, IDH2, IKZF1, KRAS, LNK, NFE2, NRAS, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, ZRSR2, BPGM, EGLN1 (PHD2), EPAS1 (HIF2A), EPOR, GATA1, GELSOLIN, HBA1, HBA2, HBB, JAK2,MPL, RUNX1, SH2B3, SRC, THPO, VHL, WAS) was employed to better characterize these diseases. Sequencing analyses of DNA from mononuclear peripheral blood cells were performed in 57/80 pts.
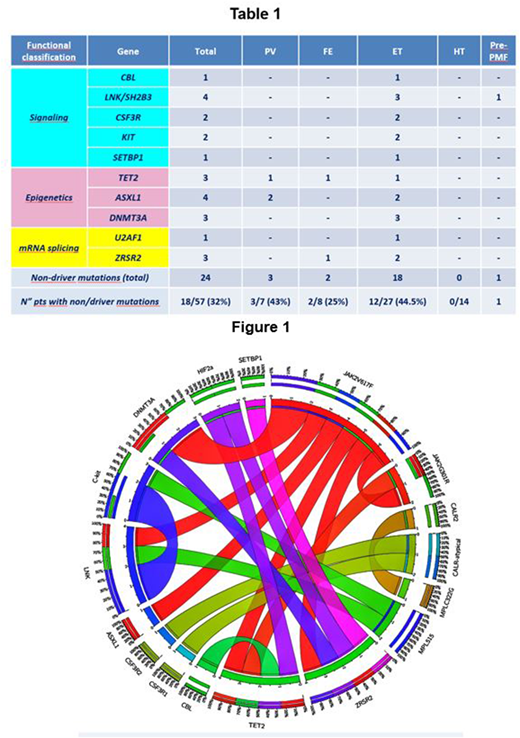
Eighty pts (M 41, F 39; median age at diagnosis: 149/12 yrs, range 3 months-1911/12 yrs), investigated by standardized methods, were retrospectively classified according to the WHO 2016 criteria as follows: 35 essential thrombocythemia (ET) (10 JAK2V617F, 2 CALR type1, 6 CALR type2, 1 CALR atypical, 16 WT), 9 polycythemia vera (PV) (4 JAK2V617F, 5 WT) and 3 primary myelofibrosis (PMF) (1 JAK2V617F, 2 WT). Twenty-three pts with MPLS505N or MPLV501A mutations and 10 pts with HIF mutations (3 pts) and/or anamnestic criteria of FE (7 WT) were considered HT and FE, respectively. The NGS-based 44-gene panel was applied to 57 MPD pts (11 JAK2V617F, 6 CALR, 12 MPLS505N, 2 MPLV501A, 3 HIF2α and 23 WT). According to the WHO 2016 criteria, 27 pts were ET, 14 HT, 8 FE, 7 PV and 1 PMF. By using the NGS panel, clonal markers were found in 12/23 (52%) pts with MPN WT: HBB and PDH2 in 2 FE, MPLW515_P518>KT in 1 ET pt and non-driver mutations in 9 pts (7 ET, 1 PF and 1 PV). Furthermore, two non-canonical driver mutations, MPLC322G and JAK2G301R were identified in 1 CALR type2 ET and in 1 JAK2V617FPV, respectively. An additional MPLV501M mutation was found in 1 MPLS505N HT. Taken together, among the 57 pts 18 (32%) had one (11/18=68%) or two (7/18=39%) non-driver mutations. Eight of the 34 pts (23.5%) with a clonal marker had additional non-driver mutations, that was single in 6 pts. Within the familial MPD, a single non-driver mutation was found in 3/8 FE pts (37.5%), while no mutations were detected in HT pts. Considering the functional classification of non-driver mutations, we found mutations in signaling (CBL, LNK/SH2B3, CSF3R, KIT, SETBP1) and splicing (U2AF1, ZRSR2) genes in ET and PMF pts, and mutations of epigenetic regulation genes (TET2, ASXL1, DNMT3A) in PV, FE and ET pts (Table 1). The co-occurrence of driver and non-driver mutations in the same individual is illustrated in the circos plot (Figure 1).
The use of a NGS-based 44-gene panel in acquired and familial pediatric MPD enabled to identify driver and non-driver mutations, not otherwise detected by conventional methods, with a substantial proportion of MPD pts (81%) showing mutations in the genes analyzed. Interestingly, we found additional neoplastic mutations in some pts with FE. Although the utilized NGS-based panel proved useful to better characterize children and adolescents with MPD, 19% of our pts still remain without any identified clonal marker. Further targeted NGS and whole genome sequencing may enable to better define MPD children without molecular markers.
Malaspina:Sapienza University, Rome: Other: Resident in Hematology. Foà:ABBVIE: Other: ADVISORY BOARD, Speakers Bureau; CELGENE: Other: ADVISORY BOARD, Speakers Bureau; AMGEN: Other: ADVISORY BOARD; INCYTE: Other: ADVISORY BOARD; NOVARTIS: Speakers Bureau; ROCHE: Other: ADVISORY BOARD, Speakers Bureau; GILEAD: Speakers Bureau; JANSSEN: Other: ADVISORY BOARD, Speakers Bureau; CELTRION: Other: ADVISORY BOARD.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal